Titration Fundamentals
A titration can be used to determine the unknown concentration of a substance (the “analyte”) by slowly adding measured quantities of another substance (the “titrant”) which reacts with the analyte in a known proportion. There are several different types of titrations, including acid-base, redox, precipitation, and complex-formation. The titration endpoint can be signaled by an added color-changing substance, an electrical property of the solution, or a visible change to the titrant or analyte. The endpoint corresponds to or is very close to the “equivalence point,” the point at which all of the analyte and titrant have reacted, and the volume of titrant used to reach the endpoint can then be used to calculate the analyte concentration.







Demos + Pricing
Learn more about our courses, get pricing, and see our platform.
Course Details
Learning Objectives
Define the terms “titrant,” “analyte,” “standard solution,” “endpoint,” and “equivalence point”
Identify the variables of the titration equation, and demonstrate how to calculate an unknown concentration based on titration results
In an acid-base titration, select a color-changing indicator based on the equivalence point pH
Describe acid-base, redox, precipitation and complex-formation titrations
Describe potentiometric, conductometric, amperometric and coulometric titration methods
Identify when a back titration might be appropriate
Describe how the titration method is chosen
Specs
Frequently Asked Questions
Why is it important to add the titrant solution in small quantities during a titration?
When can a precipitation titration be used?
In the calculation that is used to determine the concentration of the analyte solution, what is the “M”?
What is a redox titration?
What kind of titration can be used to determine the concentration of metal ions in a solution?
Sample Video Transcript
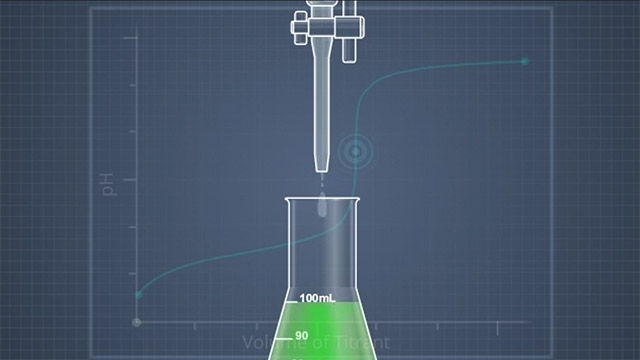
If you were to plot pH versus the volume of added titrant, you would generate a pH titration curve. The equivalence point at which the acid and base have neutralized each other occurs at the midpoint of the steepest part of this curve. This is why the titrant is added slowly and in small quantities, in order to catch the steep part of the curve and not jump over it. A pH meter can be used to monitor the pH and identify the equivalence point or an indicator substance that exists in both acid and base forms, and changes color at or near the equivalence point pH can be used. When selecting an indicator, it is important to know the color change pHs of all available indicators.